Battery Brings a Sustainable Future Closer with Every Breath

A potentially transformative energy storage solution is being developed by a group led by Yet-Ming Chiang, professor at the Department of Materials Science and Engineering at Massachusetts Institute of Technology (MIT), and Zheng Li, assistant professor of mechanical engineering at Virginia Tech.
Their research was published in the journal “Joule” in the article “Air-Breathing Aqueous Sulfur Flow Battery for Ultralow-Cost Long-Duration Electrical Storage.”
Cheap, Scalable and Powerful
This “breathing” flow battery’s most powerful benefit is that it has a low installation cost, according to the researchers. This makes it ideal for long-term storage that is needed by electrical grids.
According to the Energy Storage Association, a flow battery is a battery where the electrode components are flowing liquids separated by a membrane instead of being the solid materials that are used by most batteries.
Not only does the flow battery use environmentally friendly byproducts, it also has lower production and maintenance costs, the researchers said.
The advantages of flow batteries include their scalability and simplicity, the researchers said. The flow battery has a high degree of scalability, allowing the battery to meet larger energy demands.
When compared to normal batteries, flow batteries are more flexible because of their scalable architecture. The researcher said that to store more energy, the batteries only need larger tanks and possibly larger reactor surface areas. Also, the costs of using a larger tank and reactor surface area do not increase linearly.
In addition, flow batteries are free of the geological limitations that restrict the scaling of traditional energy storage technologies such as hydroelectricity and compressed air, the researchers said. Flow batteries are simple in design and are designed for significant long-term storage. Hydroelectricity needs several water sources and a developed infrastructure, while compressed air power uses gas, pumping and storage.
The feasibility of scaling up flow batteries increases when low-cost reactants are used.
In fact, this is the key advantage of the team’s invention. According to a member of the research team, the sulfur is a low-cost waste byproduct from the oil-refining industry, while water and air are both nearly free resources. This increases the ease of implementing such technology in the real world.
“We looked for energy storage chemistries that are super-abundant, low-cost, and yet have the capability to store a high density of electrical energy and were inspired by the potential that sulfur offered,” Chiang said.
The research team said their staff, in collaboration with the Joint Center for Energy Storage Research (JCESR), one of the largest energy storage-research centers in the world, employed unique technological and economic models to determine the best and cheapest reactant pairs.
The best use for this invention would be in combination with wind and solar power. Solar and wind technologies cannot produce constant and consistent power as a result of their dependence on variable energy sources.
Solar and wind energy have costs that arise from this intermittency. For solar and wind to compete with conventional energy, they must become reliable resources. Efficient, low-cost energy storage provides a solution to this issue by storing energy generated by solar panels and wind turbines for long periods, granting people access to power even when the turbines and panels themselves are not at work.
This is why it may be a game changer that the team’s invention with its ease of scalability, lack of geological constraints, and ultra-low cost could mean an energy revolution is one flow battery away.
Reactions and Chemistry
To understand the potential impacts of this invention, it can be helpful to understand the science behind it.
Most typical batteries work through the use of chemical reactions known as reduction-oxidation reactions. According to Steven and Susan Zumdahl’s textbook, “Chemistry,” reduction-oxidation reactions involve the exchange of electrons and ions from one chemical to another. With these electrons, an electrical current can be created.
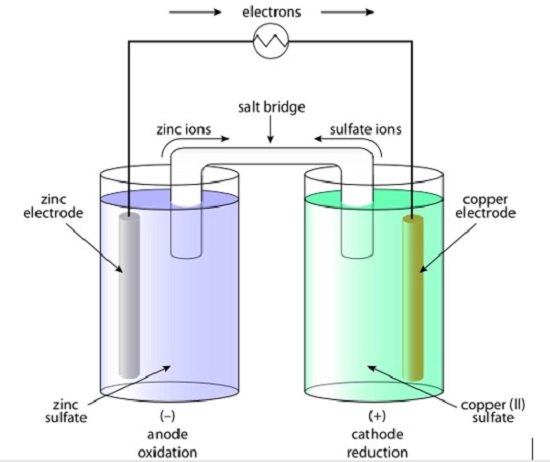
Galvanic Cell. (Source: Galvanic Cell, Wikipedia. Creative Commons License)
In an electrochemical cell, a solid electron-donating electrode is put in a liquid salt mixture while an electron-receiving electrode is put in another liquid. As the book explains, when a wire is connected to both electrodes, electrons move from one side to the other, leading to an electric current. Electrons move from the anode to the cathode.
When the electrons move, the anode becomes negatively charged and the cathode becomes positively charged. The book also shows that in order to balance this, a bridge made of salts is usually connected to both cells. That way, electrons and ions from the salts can move to the anode and cathode.
In a battery, the electrodes are in the body of the battery. When a circuit is connected to the battery, electrons can move and the reduction-oxidation reactions take place. Once all of chemicals have been used up, the battery is dead.
The battery invented by the research team uses liquids instead of solid electrodes. According to Corrosionpedia and the textbook, a flow battery’s anolyte solution lies at the anode and donates electrons, while the catholyte solution accepts electrons at the cathode. The battery’s key component is a liquid polysulfide solution, which forms the anolyte. The catholyte solution used in this battery was either lithium sulfate or sodium sulfate.
When charging the battery, the catholyte goes through an oxygen-evolution reaction. This means that oxygen is made, then released into the air. The lithium and sodium ions then travel through a membrane to balance the extra electric charge in the anolyte.
During discharging, the opposite occurs: the sulfur loses electrons, leading to an electric current through the circuit. The lithium and sodium move back to the catholyte, and oxygen is taken from the surrounding environment during an oxygen-reduction reaction.
The way it uses oxygen in both charging and discharging gives this battery the advantage of making environmentally friendly byproducts while saving electrical energy.
Join our LinkedIn group to discuss this article. You may also email the author directly using our contact form.